slim6y
Almost Legendary
Where does our carbon come from? And what is a radioisotope anyway?
Reference for Bluetonguey bungy 1
So - what is this stuff we call carbon?
Carbon is awesome! Really really really awesome.
Let's take some basic facts about carbon first.... Blah blah blah... 6.... blah blah..... chain.... blah blah.... Let's assume you've scaffolded yourself to a Bluetongue1 equivalence.
I don't plan on reinventing the wheel, but I do plan on clearing up misconceptions etc and perhaps then allow myself to think that what i was taught had the potential to be correct.
The next lines come directly from Where Does the Carbon Come From? | Wired Science | Wired.com
Suppose you were to look all around the universe and count all the different elements. What would you find? Well, you would find a whole bunch of hydrogen and helium. But there is also quite a bit of carbon. Here is a chart of the relative abundances of the different elements from Wikipedia.
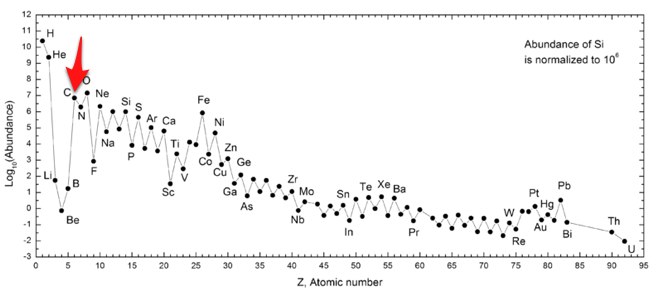
In case you didn’t notice, I put an arrow at the carbon element so you could see it. Make sure you notice one other thing. The vertical scale is a log scale. This means that there is 3 times as much hydrogen as helium. Now, for the cool part. Hydrogen and helium are obviously common. Oxygen and then carbon are the next two most abundant elements. Way more abundant than beryllium and boron even though Be and B have fewer protons than either oxygen or carbon. Oh, one more note – this chart shows the relative abundance of elements in the Milky Way, not the universe – but you get the idea.
Why is there so much carbon? I guess maybe we should start from the beginning.
I am plagiarising which is a sin... I know. But it's ok... Because what I want to say is contained on these very websites:
So to save copying anymore, let's assume you know what a proton and a neutron is - and if you don't, the proton is the positive part and the neutron is the....
Neutral part... (if you accidentally said it's the negative part, don't be ashamed, but for future reference, the electron is the negative part - however arbitrary the charge sign is).
Stellar Production of Particles
There is another place where you can get: a) very fast particles and b) very many particles very close together. In a star. This is the fusion process in our star (also known as THE SUN). First, there is the proton-proton chain. In this process, helium nuclei are created from protons. Here is a diagram from wikipedia.
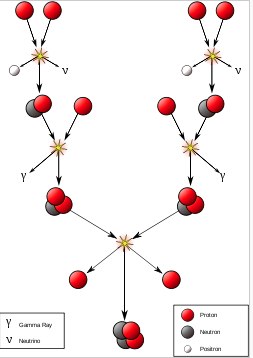
Basically, you start with 4 protons and end up with helium (and some positrons). Once the star produces enough helium, carbon can be made through the triple-alpha process.
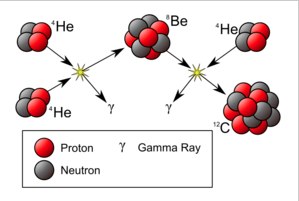
And boom. Carbon. However, there is a problem. If you look at the above reaction, it is very unlikely to occur unless the carbon-12 produced is in an excited state. Wait, can a nucleus be excited even if there are no electrons? Sure. Think of it like an oscillating ball of jello. Wait, there is one other problem. Is this excited state of carbon even possible? This is known as the Hoyle-state – a state predicted by Fred Hoyle quite some time ago.
So far - this is lovely...
Bluetongue1 indicated this "The earth is not bombarded by carbon and never was."
I'd like to just indicate now this:
"If the carbon contained in the comets and asteroids bombarding Earth during this phase existed only in the form of simple carbon molecules such as formaldehyde, it would have also quickly evaporated, Cody said."
This is taken from Space Poison Helped Start Life on Earth?
Evidence suggests that even as early as 12,000BC we were still getting bombarded by LARGE carbon carrying comets and asteroids and in fact some rocks in Greenland back that up.
Earth has been and is STILL being bombarded by carbon.
The age of the remains of plants, animals, and other organic material can be determined by measuring the amount of carbon-14 contained in that material. Carbon-14, a radioactive form of the element carbon, is created in the atmosphere by cosmic rays (invisible, high-energy particles that constantly bombard Earth from all directions in space).
Read more: Dating Techniques - humans, body, used, process, Earth, life, plants, form, energy, methods, animals, carbon, substance, plant, waves, Relative dating, Absolute dating Dating Techniques - humans, body, used, process, Earth, life, plants, form, energy, methods, animals, carbon, substance, plant, waves, Relative dating, Absolute dating
So - this suggests that the carbon is already in the atmosphere - so where did the atmospheric carbon come from - you can't tell me it's just from women gas bagging all the time. I'm pretty sure that carbon in our atmosphere (upper) must come from some other source....
Even Bill Bryson mentions the inadequacies of C-14 dating because of the continual change of C-14 in our atmosphere.
Open air nuclear testing also contributed to C-14 in out atmosphere - another form of BOMBARDMENT (after all, a nuclear bomb is a bombardment by its very nature).
Nitrogen (which is what gets smashed by cosmic rays in the outer atmosphere) comes from.... Ahem.... Probably volcanoes and many other sources - it makes up 78% of our atmosphere and... Yep... When carbon falls from the upper atmosphere - it 'bombards' the earth!!!
Golly... I think I can safely say the word bombardment (by carbon) is not incorrect - technically it was less elegant than some other word (such as (insert Bluetngue1 word here)).
So - clarity shouldn't be taken to far from reality... Bombardment is here to stay.
The rate of decay is exponential to the quantity. It is continually decreasing, not constant.
Yes Bluetongue1 - you're correct here. It is continually decreasing... at around (as I suggested) 5730 (+/- 40) years HALF LIFE!
That's why I said - constant(ish) decay!!! Geeeee.... Is this another time you could have misinterpreted the elegance of a slim6y word vs the arrogance of a Bluetongue1 word?
My guess is yep....
All materials had to be made of fusion some how.... Let's stop there because my dog wants to play... His name is Piri. He actually doesn't care where his C-14 comes fro, he just knows (because I told him) that he contains some C-14....
He's a good dog really...
Reference for Bluetonguey bungy 1
So - what is this stuff we call carbon?
Carbon is awesome! Really really really awesome.
Let's take some basic facts about carbon first.... Blah blah blah... 6.... blah blah..... chain.... blah blah.... Let's assume you've scaffolded yourself to a Bluetongue1 equivalence.
I don't plan on reinventing the wheel, but I do plan on clearing up misconceptions etc and perhaps then allow myself to think that what i was taught had the potential to be correct.
The next lines come directly from Where Does the Carbon Come From? | Wired Science | Wired.com
Suppose you were to look all around the universe and count all the different elements. What would you find? Well, you would find a whole bunch of hydrogen and helium. But there is also quite a bit of carbon. Here is a chart of the relative abundances of the different elements from Wikipedia.

In case you didn’t notice, I put an arrow at the carbon element so you could see it. Make sure you notice one other thing. The vertical scale is a log scale. This means that there is 3 times as much hydrogen as helium. Now, for the cool part. Hydrogen and helium are obviously common. Oxygen and then carbon are the next two most abundant elements. Way more abundant than beryllium and boron even though Be and B have fewer protons than either oxygen or carbon. Oh, one more note – this chart shows the relative abundance of elements in the Milky Way, not the universe – but you get the idea.
Why is there so much carbon? I guess maybe we should start from the beginning.
I am plagiarising which is a sin... I know. But it's ok... Because what I want to say is contained on these very websites:
So to save copying anymore, let's assume you know what a proton and a neutron is - and if you don't, the proton is the positive part and the neutron is the....
Neutral part... (if you accidentally said it's the negative part, don't be ashamed, but for future reference, the electron is the negative part - however arbitrary the charge sign is).
Stellar Production of Particles
There is another place where you can get: a) very fast particles and b) very many particles very close together. In a star. This is the fusion process in our star (also known as THE SUN). First, there is the proton-proton chain. In this process, helium nuclei are created from protons. Here is a diagram from wikipedia.

Basically, you start with 4 protons and end up with helium (and some positrons). Once the star produces enough helium, carbon can be made through the triple-alpha process.

And boom. Carbon. However, there is a problem. If you look at the above reaction, it is very unlikely to occur unless the carbon-12 produced is in an excited state. Wait, can a nucleus be excited even if there are no electrons? Sure. Think of it like an oscillating ball of jello. Wait, there is one other problem. Is this excited state of carbon even possible? This is known as the Hoyle-state – a state predicted by Fred Hoyle quite some time ago.
So far - this is lovely...
Bluetongue1 indicated this "The earth is not bombarded by carbon and never was."
I'd like to just indicate now this:
"If the carbon contained in the comets and asteroids bombarding Earth during this phase existed only in the form of simple carbon molecules such as formaldehyde, it would have also quickly evaporated, Cody said."
This is taken from Space Poison Helped Start Life on Earth?
Evidence suggests that even as early as 12,000BC we were still getting bombarded by LARGE carbon carrying comets and asteroids and in fact some rocks in Greenland back that up.
Earth has been and is STILL being bombarded by carbon.
The age of the remains of plants, animals, and other organic material can be determined by measuring the amount of carbon-14 contained in that material. Carbon-14, a radioactive form of the element carbon, is created in the atmosphere by cosmic rays (invisible, high-energy particles that constantly bombard Earth from all directions in space).
Read more: Dating Techniques - humans, body, used, process, Earth, life, plants, form, energy, methods, animals, carbon, substance, plant, waves, Relative dating, Absolute dating Dating Techniques - humans, body, used, process, Earth, life, plants, form, energy, methods, animals, carbon, substance, plant, waves, Relative dating, Absolute dating
So - this suggests that the carbon is already in the atmosphere - so where did the atmospheric carbon come from - you can't tell me it's just from women gas bagging all the time. I'm pretty sure that carbon in our atmosphere (upper) must come from some other source....
Even Bill Bryson mentions the inadequacies of C-14 dating because of the continual change of C-14 in our atmosphere.
Open air nuclear testing also contributed to C-14 in out atmosphere - another form of BOMBARDMENT (after all, a nuclear bomb is a bombardment by its very nature).
Nitrogen (which is what gets smashed by cosmic rays in the outer atmosphere) comes from.... Ahem.... Probably volcanoes and many other sources - it makes up 78% of our atmosphere and... Yep... When carbon falls from the upper atmosphere - it 'bombards' the earth!!!
Golly... I think I can safely say the word bombardment (by carbon) is not incorrect - technically it was less elegant than some other word (such as (insert Bluetngue1 word here)).
So - clarity shouldn't be taken to far from reality... Bombardment is here to stay.
The rate of decay is exponential to the quantity. It is continually decreasing, not constant.
Yes Bluetongue1 - you're correct here. It is continually decreasing... at around (as I suggested) 5730 (+/- 40) years HALF LIFE!
That's why I said - constant(ish) decay!!! Geeeee.... Is this another time you could have misinterpreted the elegance of a slim6y word vs the arrogance of a Bluetongue1 word?
My guess is yep....
All materials had to be made of fusion some how.... Let's stop there because my dog wants to play... His name is Piri. He actually doesn't care where his C-14 comes fro, he just knows (because I told him) that he contains some C-14....
He's a good dog really...
Last edited:





